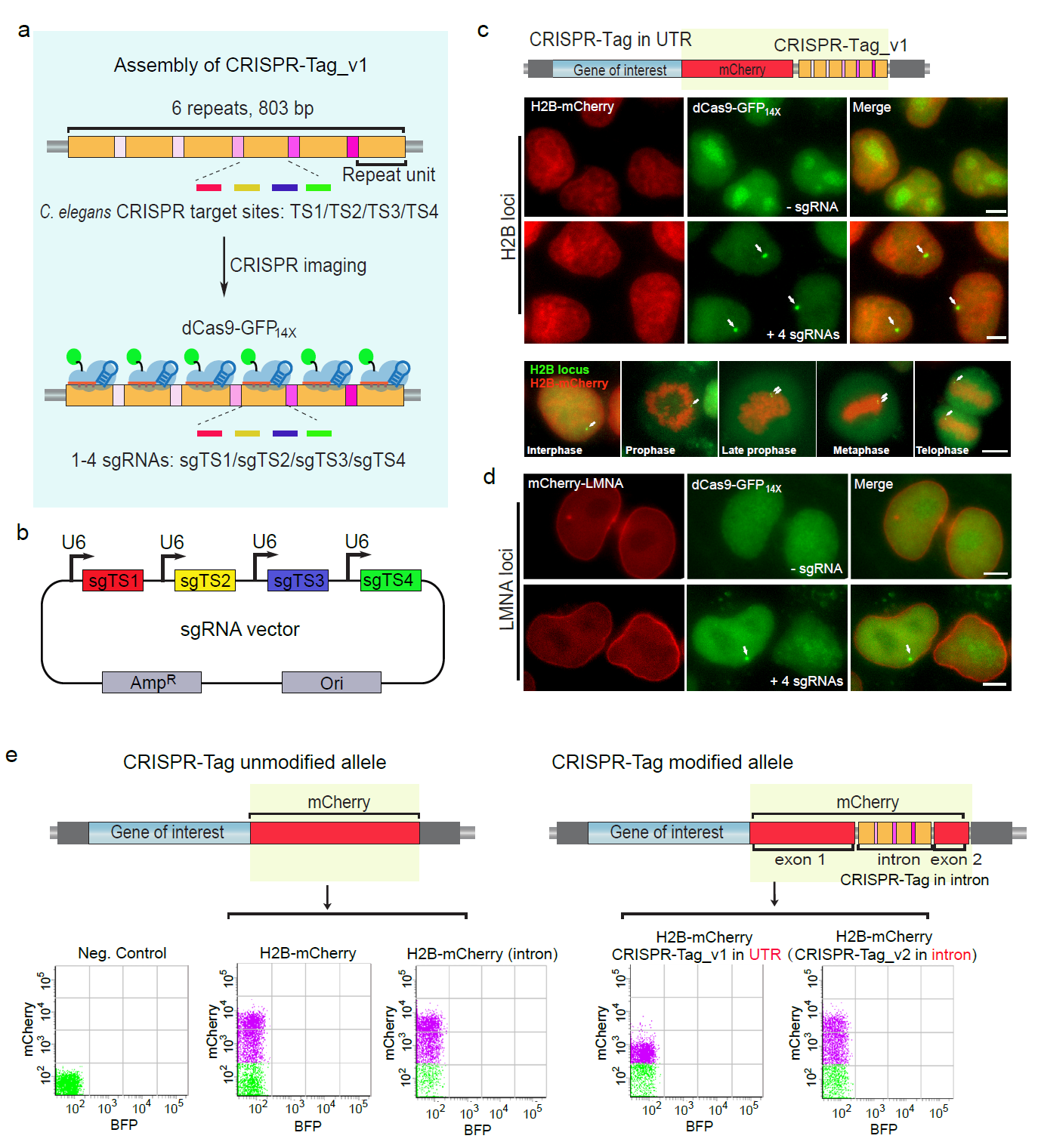
On November 29th, 2018, Dr. Baohui Chen’s group published a research article entitled “Efficient labeling and imaging of protein-coding genes in living cells using CRISPR-Tag” in Nature Communications. In this article, the authors develop a CRISPR-Tag system using one to four highly active sgRNAs to specifically label protein-coding genes with a high signal-to-noise ratio for visualization by wide-field fluorescence microscopy.
The catalytically dead version of Cas9 (dCas9) has been extensively explored for imaging endogenous genomic loci in living cells. However, most of targets visualized by dCas9 system are still limited to repetitive genomic region. The major challenge is, when targeting non-repetitive genomic regions, it requires multiple sgRNAs function simultaneously to provide a sufficient signal-to-noise ratio for microscopy detection. The lack of efficient tools to image non-repetitive genes in living cells has limited our ability to explore the functional impact of the spatiotemporal dynamics of such genes. Dr. Chen’s group addressed this issue by developing new type of DNA tags, termed “CRISPR-Tag”, to label endogenous protein-coding genes in living cells at the level of single cells and single loci. This approach involves assembling the CRISPR-Tag within the intron region of a fluorescent protein and then integrating this cassette to N- or C-terminus of a specific gene, which enables simultaneous real-time imaging of protein and DNA of human protein-coding genes. Most importantly, the use of CRISPR-Tags does not affect gene expression. All CRISPR-Tags they created are smaller than 850 bp. Thus, the CRISPR-Tag system represents an efficient, easy and scalable DNA tagging system in living human cells.
In theory, CRISPR-Tag approach can be applied broadly for any other species, such as using Human CRISPR-Tag for C. elegans genomic loci labeling. Furthermore, a same set of CRISPR-Tag and sgRNAs is applicable for tagging any genomic loci. This is an advantage over previous CRISPR imaging technique which requires at least 26 sgRNAs. Individual genes and genomic regions locate at different positions in the three-dimensional space of the nucleus. The long-standing questions are whether the position of a gene affects its activity and how the gene positioning is maintained and regulated. There is no doubt that utilizing live-cell DNA imaging techniques which allow direct visualization of gene positioning and gene expression in living cells simultaneously, the researchers will be able to uncover how gene position is linked to gene activity.
Dr. Baohui Chen (Department of Cell Biology and Bone Marrow Transplantation Center of the First Affiliated Hospital, Zhejiang University School of Medicine) is the first author of this paper. Dr. Baohui Chen and Dr. Bo Huang (University of California, San Francisco) are the co-corresponding authors.